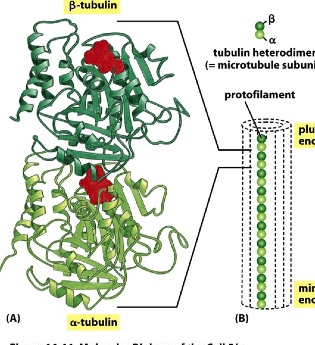
Microtubule
- subunit: tubulin — two kinds forming a dimer: alpha(-) and beta(+) tubulin
- the dimer is formed through non-covalent association
- each subunit can bind to GTP, and due to the asymmetrical position of GTP, it’s polar—> making a plus side and a minus side
- 2 tubulin subunits form a dimer —> many dimers form a protofilament —> 13 protofilaments form a long hollow microtubule
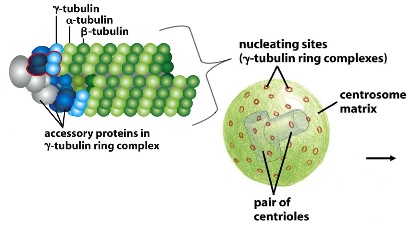
Gamma tubulin nucleates the protofilaments together. It forms a gamma tubulin ring complex with other accessory proteins. Without these, the protofilaments would fall apart. A pair of centrioles and hundreds of proteins form the centrosome matrix, which has nucleating sites on its surface. These ring complexes bind to the minus side of the tubules, and have the plus side radiating away from the middle of the MTOC (microtubule-organizing centre)
microtubules undergo dynamic instability (+): continuous growing and shrinking. This allows the microtubules to explore 3D space around themselves and search for targets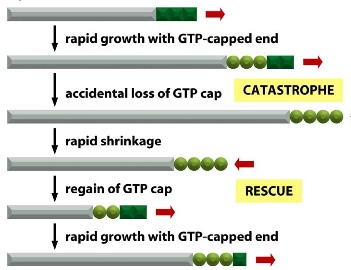
- growing: led by GTP-bound subunits (T form) —> starting polymerization
- shrinking: caused by GTP hydrolysis (GDP-bound subunits: D form)
- when there’s only GDP-bound subunits, the tubule falls apart 100 times faster than when bound to GTP cap
- if D forms are created faster than T form, then catastrophe occurs; if T forms are added faster than D form, the growth is rescued
microtubules can form their own co-ordination system by having the growing tubules pushing against the plasma membrane, so eventually when there’s equal force pushing on all four sides, the centrioles can move to the centre (other regulatory proteins are needed too). This system allows for the transport of cargo within the cells. But microtubule network has the potential to be completely rearranged, such as the case during cell division. Centrosomes are typically close to the nucleus.
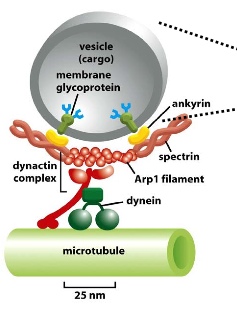
Transport with Motor Proteins
2 motor proteins can bind to microtubules and carry vesicles to either end of the tubule:
- dynein moves to the minus end (-)
- kinesin moves to the plus end (+)
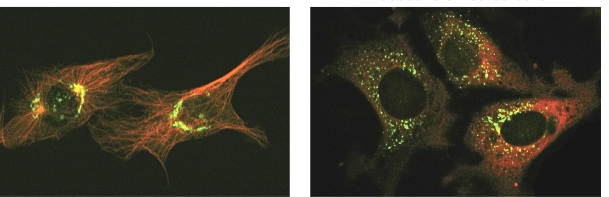
Experiment 1: disrupting microtubules to see what happens to the localization of Golgi
Golgi is originally near the nucleus, with centrosomes in the centre. After disruption, Golgi flows in all directions and are unable to concentrate on the minus end.
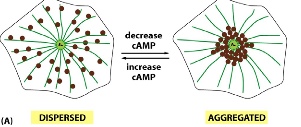
Experiment 2: observing how African cichild fish change colour
The fish can change their colour from dark (pigment dispersed for all the cells) to light (pigments concentrated in the middle to form spots). When the fish appears dark, kinesin and dynein compete for the cargo (containing the pigment filled melanosomes), but neither is winning —> pigments are neither going to the plus end nor the minus end. When the fish appears light, kinesin is inhibited, allowing dynein to carry the pigments to the minus end, thereby concentracting the pigments.
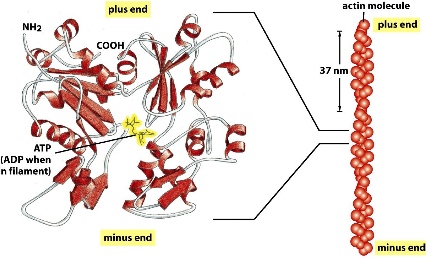
Actin filament
- monomer, also polar, ATP-bound
- there are only two protofilaments formed with subunits connecting head to tail and twisting around each other
- hydrolysis of ATP leads to dissociation of the subunits
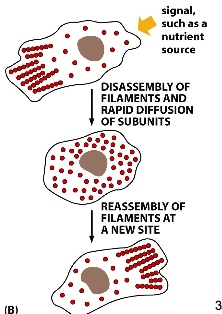
- ratio of assembly vs. dissociation determines whether the filament grows or shrink
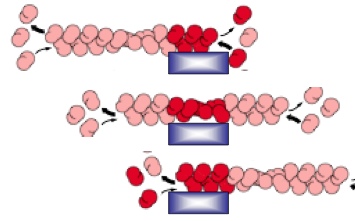
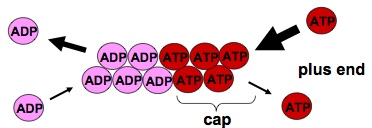 actin filaments grow at the end where ATP comes in
actin filaments grow at the end where ATP comes in
Treadmilling: net assembly at the plus end, ant net dissociation at the minus end. So that a flux of ctin subunits can allow the actin filaments to maintain its length
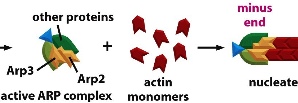
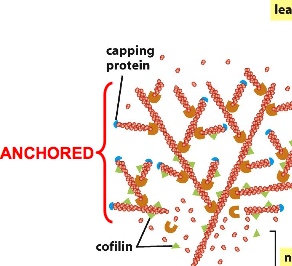
ARP(actin-related protein) complex nucleates actin filaments so they don’t easily fall apart. ARP complex can be turned on by activating factor. The ARP complex acts as the plus end so that actin subunits can add on to it using the minus end. ARP complexes can also bind to existing filaments forming 70 degrees, forming a network. The constant dissociation of the minus end and building of the plus end can direct cell movement
The movement of actin filament can be controlled by attaching actin to stationary anchor. So when one end shrinks while another end grows, the net effect is that the filament moves in one direction.
Where are these anchors found?
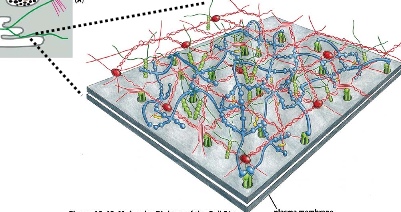
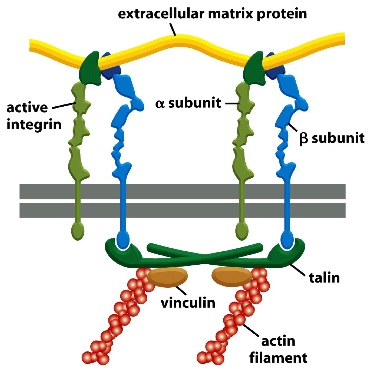
The plasma membrane of an intracellular organelle. The extracellular matrix proteins form the stable anchors.
The cross-section shows that the alpha and beta subunits of integrin proteins(transmembrane heterodimers with non-covalent association) can connect extracellular matrix proteins with actin filament through an adaptor protein.
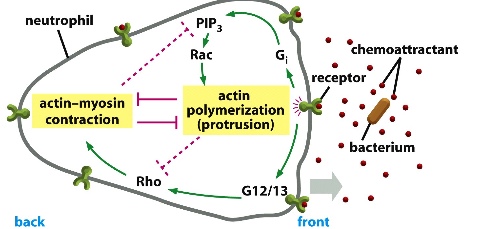
Chemoattraction
The immune cells can detect molecules (signals) secreted by bacteria in the extracellular space. The bacteria produces a gradient of chemicals (most concentrated at its source). Surface receptors on the immune cells recognize the signals and send signal to activate actin polymerization in the direction of the bacteria while having the opposite side contracted (with myosin).
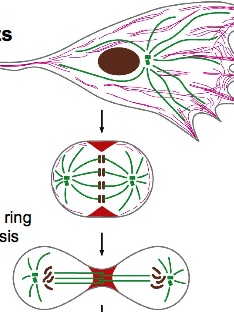
Cells can also have their actin network completely rearranged: such as during cell division where the entire network is dissembled, then forming a contractile ring at the equator.


 actin filaments grow at the end where ATP comes in
actin filaments grow at the end where ATP comes in